|
⇤ ← Revision 1 as of 2019-05-23 20:08:08
Size: 1296
Comment: extra e2tomo functions
|
Size: 4129
Comment: start on focused refinement
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 4: | Line 4: |
== Focused refinement == Refine local regions of a large complex. Available after 05/23/2019. Still under development. * Start from a successful subtomogram refinement of the full complex (spt_xx folders). For large complexes, it is more convenient to run the full complex refinement with bin2 or smaller particles, then run local refinement with unbinned particles. Here we start from the result from the ribosome tutorial dataset: [[https://blake.bcm.edu/emanwiki/EMAN2/e2tomo | e2tomo]]. * Here we follow the [[https://doi.org/10.7554/eLife.36861.001 | Relion]] single particle multibody refinement paper and focus on the "head" part of the ribosome. From the current subtomogram averaging result, it is quite obvious that the head part has a worse resolution. * First we need to locate the region of interest. Here we use the filter tool to do it interactively. Run '''Fitertool''' on the 3D structure we currently have and insert a `mask.soft` processor. Play with the parameters. Here I have dx=32:dy=32:dz=0 and the radius of the head part is roughly 36 pixels. {{attachment:filtertool0.png| Locate region of interest |width=400}} * Now clip the region out and take a look. The box size of the local region needs to be at least 72, but we use 128 regardless because extra padding is always good. Run {{{ e2proc3d.py spt_01/threed_05.hdf ref_small_0.hdf --clip 128,128,128,128,128,96 }}} Here the first three numbers after `--clip` are the box size in x,y,z, and the next three numbers are the center of the box. My original box size is 192, so the center is at 96,96,96. Since we decided that the shift is 32,32,0 in the previous step, the new center should be at 128,128,96. * Next we need to make a mask of the region. Open '''Filtertool''' on the map we just generated (`ref_small_0.hdf`), and make a mask you like. Try making the mask as soft as possible but do not touch the edge of the box. Here I use a `mask.soft` with `mask.auto3d.thresh`. Once you are satisfied, save the map from '''Filtertool''' in '''File -> Save processed Map'''. The map will be saved as `processed_map.hdf`, and we rename it to `mask_small_0.hdf`. Make sure you save the mask with values between 0 and 1, not the masked map. {{attachment:filtertool0.png| Locate region of interest |width=400}} * We also need to prepare the transform input for particle extraction. Open a new text file and write {{{ {'type':'eman', 'tx':32, 'ty':32, 'tz':0} }}} Save the file as `xf_small.txt`. This seems overcomplicated because this framework also allows local refinement of multiple asymmetrical units individually. For details on how to do this, check the [[https://blake.bcm.edu/emanwiki/EMAN2/e2tomo_more | next section]]. == Focused refinement on multiple asymmetrical units == |
|
| Line 14: | Line 42: |
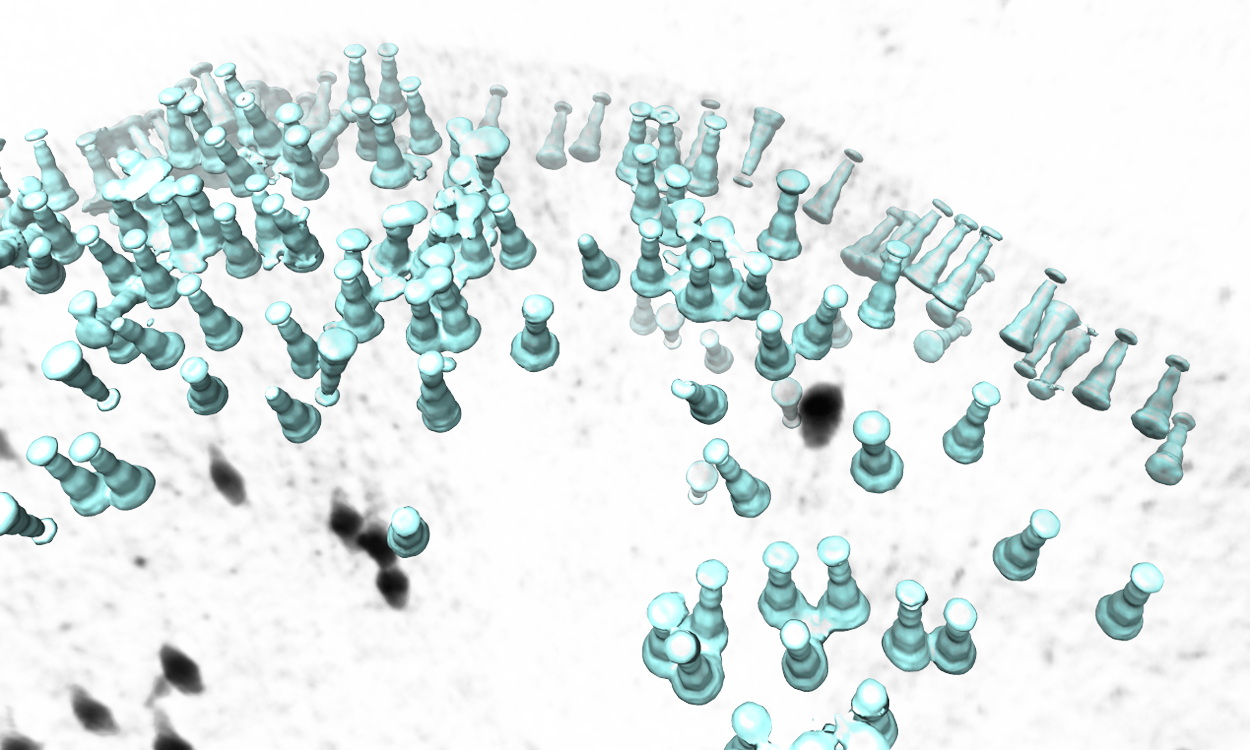
| The program will then find all particles in the selected tomogram that are used in the refinement, map the averaged structure back, and produce a file called ''ptcls_in_tomo_xx_yy.hdf'', where ''xx'' is the name of tomogram and ''yy'' is the number of iteration used. This is sometimes useful for objects in cellular environment but is quite uninteresting in this ribosome dataset. Image rendered with Chimera. | The program will then find all particles in the selected tomogram that are used in the refinement, map the averaged structure back, and produce a file called ''ptcls_in_tomo_xx_yy.hdf'', where ''xx'' is the name of tomogram and ''yy'' is the number of iteration used. This is sometimes quite useful for objects in cellular environment (when membrane proteins are obviously upside down for example). Image rendered with Chimera. |
| Line 16: | Line 44: |
| {{attachment:map_ptcls_to_tomo.png| Map particles to tomograms |width=600}} | {{attachment:map_ptcls_to_tomo.png| Map particles to tomograms |width=600}} |
Extra functions for EMAN2 tomography
- This page describes extra functionalities of EMAN2 tomography workflow. This tutorial is frequently updated, so it is better to have EMAN2 version as new as possible.
Focused refinement
Refine local regions of a large complex. Available after 05/23/2019. Still under development.
Start from a successful subtomogram refinement of the full complex (spt_xx folders). For large complexes, it is more convenient to run the full complex refinement with bin2 or smaller particles, then run local refinement with unbinned particles. Here we start from the result from the ribosome tutorial dataset: e2tomo.
Here we follow the Relion single particle multibody refinement paper and focus on the "head" part of the ribosome. From the current subtomogram averaging result, it is quite obvious that the head part has a worse resolution.
First we need to locate the region of interest. Here we use the filter tool to do it interactively. Run Fitertool on the 3D structure we currently have and insert a mask.soft processor. Play with the parameters. Here I have dx=32:dy=32:dz=0 and the radius of the head part is roughly 36 pixels.
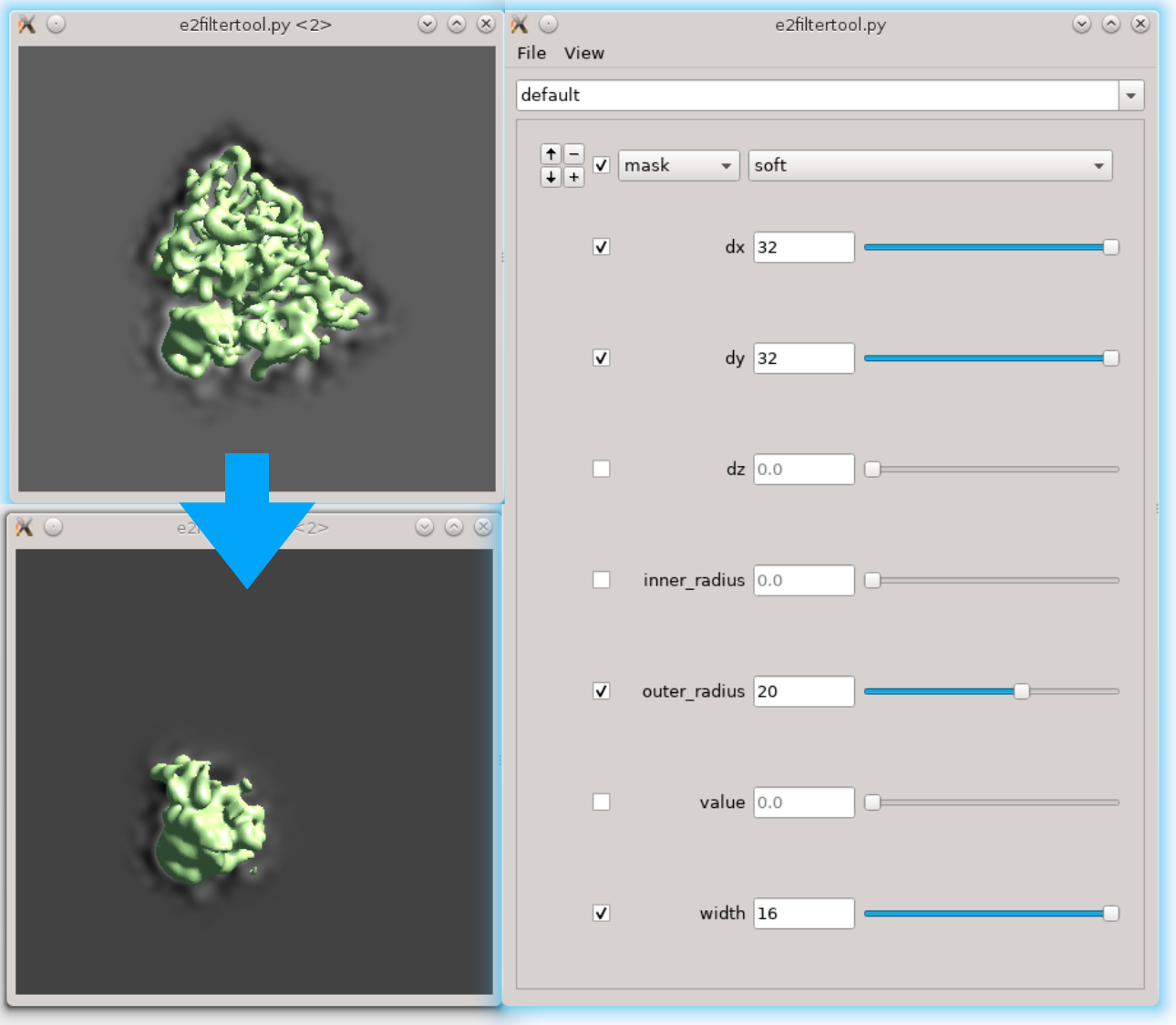
- Now clip the region out and take a look. The box size of the local region needs to be at least 72, but we use 128 regardless because extra padding is always good. Run
e2proc3d.py spt_01/threed_05.hdf ref_small_0.hdf --clip 128,128,128,128,128,96
Here the first three numbers after --clip are the box size in x,y,z, and the next three numbers are the center of the box. My original box size is 192, so the center is at 96,96,96. Since we decided that the shift is 32,32,0 in the previous step, the new center should be at 128,128,96.
Next we need to make a mask of the region. Open Filtertool on the map we just generated (ref_small_0.hdf), and make a mask you like. Try making the mask as soft as possible but do not touch the edge of the box. Here I use a mask.soft with mask.auto3d.thresh. Once you are satisfied, save the map from Filtertool in File -> Save processed Map. The map will be saved as processed_map.hdf, and we rename it to mask_small_0.hdf. Make sure you save the mask with values between 0 and 1, not the masked map.

- We also need to prepare the transform input for particle extraction. Open a new text file and write
{'type':'eman', 'tx':32, 'ty':32, 'tz':0}
Save the file as xf_small.txt. This seems overcomplicated because this framework also allows local refinement of multiple asymmetrical units individually. For details on how to do this, check the next section.
Focused refinement on multiple asymmetrical units
Map particles to tomograms
There is a simple tool to map the averaged structure to the determined position and orientation of each particle in a tomogram. Available after EMAN2.3. In versions after 05/23/2019, the function is moved to the Analysis and Visualization section in the GUI.
Subtomogram Averaging -> Map particles to tomograms
Set path to be one of the spt_XX folder (not the subtlt ones).
Set iter to be the iteration you want to use from the refinement.
- Browse for one tomogram you want to map the particles to.
The program will then find all particles in the selected tomogram that are used in the refinement, map the averaged structure back, and produce a file called ptcls_in_tomo_xx_yy.hdf, where xx is the name of tomogram and yy is the number of iteration used. This is sometimes quite useful for objects in cellular environment (when membrane proteins are obviously upside down for example). Image rendered with Chimera.